Europe confirmed the importance of cross-sectorial and cross-country cooperation by launching the new Innovative Health Initiative (IHI) building on the shoulders of two giants: the successful Innovative Medicines Initiative (IMI) 1 and 2.
In this article we provide you with five insights on the best practices to continue delivering value beyond EU funding.
Sustainability strategy
With a total of more than €7.6 billion euro, the EU has been investing in ambitious projects, carried out by large public-private consortia that are expected to develop concrete outcomes and create impact in the Life Science and Health (LSH) sector. How do we guarantee that the results in projects continue to add value and are harnessed and implemented beyond the funded period?
This is a crucial question that should be addressed early in developing the IHI proposal, as the real impact of a project relies on the strategy for valorising its outputs, also known as the sustainability strategy.
At ttopstart, we support leading researchers and innovative companies in the field of LSH to change patients’ lives for the better. ttopstart’s consultants are involved in IMI & IHI projects from start to end: with our twelve years of experience in the field, we support IMI & IHI projects by co-developing strong proposals that consider how to sustain the results in the future. Here we provide five insights on the best practices to continue delivering value beyond the EU funding. We integrated our expertise with results from a study we conducted by interviewing the project coordinators and leaders of eight IMI (1 or 2) projects.
In August 2022, there were approximately 17 continued IMI projects. These projects include GetReal & GetReal Initiative, SUMMIT, U-BIOPRED, SafeSciMET, PharmaTrain, EU2P, OpenPHACTS, OncoTrack, K4DD, ADVANCE, CANCER-ID, EUPATI & EFOEUPATI, RADAR-CNS & AD, EBOVAC, EQIPD, RAPID-COVID and eTRICKS (Table 1).
“A good sustainability strategy is crucial to enable a project to deliver impact and value beyond the funding period.”
DELIELENA POLI –
CONSULTANT AT TTOPSTART






























Table 1. List of IMI projects that ended before May 2022 and continued with a successor entity.
Insight 1: Shared benefits and risks
Academia and pharma companies should be equally heard and have an equal division of roles throughout the project phase. Additionally, the benefits of all parties, both within, and outside the project time frame should be defined at an early stage. IMI and IHI projects are characterised by cooperation between the public and private sector and across disciplines. IHIs favour connections between parties from Pharma, Medtech, BioTech, AI, imaging, and patients. If the discussion around the involvement beyond the project is started in the proposal phase, it is easier to get parties onboard for the sustainable future of a project. In particular, the gains of big pharma players (e.g. work pre-competitively to address gaps in the pharma value chain1) should be clear from the beginning of the project. Pharma companies are crucial for decision making on the sustainable options because of their expertise and large fund availability, thus, if their gains are delineated, trust can be built along with willingness to invest in the project continuation.
Insight 2: Follow up project vs. a new legal entity
There are several routes an IMI/IHI project can take, depending on the interests of those on board. The three main legal structures include an association, a foundation, or an entity part of a university (e.g., a department). Other forms are possible, such as non-profit charities, start-ups/spin-offs, or even a new collaborative project. The governance structure of a private non-profit foundation is one of the easier successor legal forms to set up, nonetheless, depending on the topic of the project other alternatives might be preferred. If the main topic of the IMI/IHI project is fundamental research, with an output that is difficult to commercialise, it is best to consider a follow-up IHI project as a sustainable option.
Insight 3: The structure of the successor organisation
Some funding options are more fitting than others depending on the successor entity structure, hence the importance of deciding on the structure of the successor organisation as soon as possible. Financing is crucial to ensure for sustainable project results. Sustainable funding options include membership fees and sponsorships for associations, foundations and charities, and services for a fee for spin-offs1. A combination of the membership fee and service offering revenue is also possible depending on the structure of the sustainable form. Membership fees are the most popular funding model although it is difficult to justify the fees. Fees reflecting the different levels of engagement depending on the members needs and interests have become the standard in associations and foundations2. The sooner IMI and IHI projects realise whether they need to establish membership fees, the better, as the challenge is deciding on a pricing level taking into consideration the differences between academia, industry and SME’s.
Insight 4: NL top country, Belgium second place
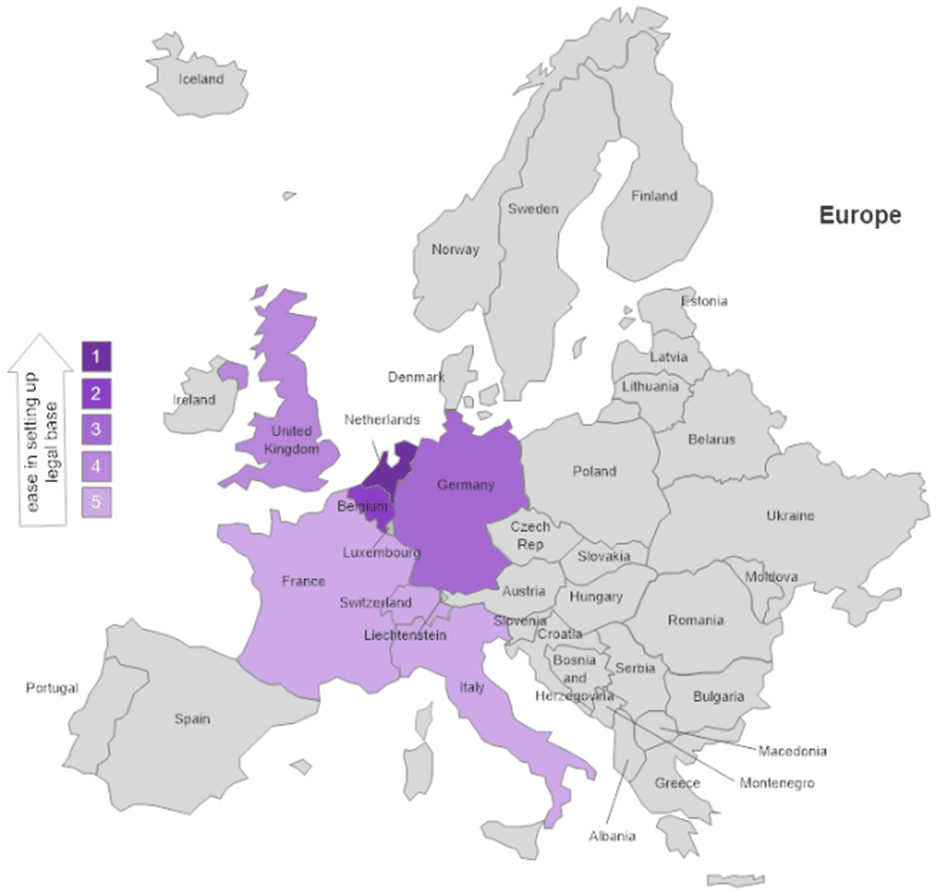
Based on the discussions with the eight project coordinators, the preferred country for setting up the legal base of a sustainable form is the Netherlands (Figure 1). The interviewees from sustainable forms set up in The Netherlands noticed ease in the procedures for setting up both companies (start-ups) and associations or foundations compared to other companies. The UK used to be a particularly favourable location for charities, but the procedures became more complex after Brexit. Belgium and Germany are good options for the legal base of an association although the bureaucratic procedures for foundations and start-ups can be complicated in Germany. Finally, Italy, France and Switzerland have complicated administrative requirements, especially for new companies.
Figure 1. Ease in setting up the legal base of a successor sustainable entity.
Insight 5: The importance of a clear developed business plan
Having a clear, structured business plan that is approved by all partners can aid the setup and speed up the process of setting up a sustainable form. With a carefully thought-out document that contains the company structure, an overview of external key partners and a funding roadmap after the project, this will ensure for a smooth transition between the end of the project and the start of the sustainable form. A work package dedicated to sustainability will ensure that sustainability is focused on from the start of the project.
Future prospects – needs identified by project coordinators
- The sustainability of IMI and IHI projects would benefit from the involvement of younger generations from the start. IMI projects need funding and an established infrastructure and workforce willing to continue in the long-term. Often there is a large number of seniors, especially in the higher governance level, which clearly guarantees experience and increases the chances of receiving approval. Nonetheless, some of the interviewees claimed that “It is good to involve the younger generations too”.
- Some coordinators would suggest the EU to develop funds to sustain promising projects. The solidity of the network of partners in IMI and IHI projects is at the core of a good sustainability strategy, however, there is the need for administrative and financial support. The EU could promote project sustainability by rewarding extra financial support the projects with outstanding sustainable potential or results.
- Some of the interviewees developed the idea of a financial support system for IMI and IHI projects even further. Those projects that are successful and sustain themselves generating revenues could be matched with the recently ended projects that are seeking financial support. As such this matching tool/platform would 1) leverage synergies between EU funded projects and 2) partly solve the issue of having no financing means beyond the project period.
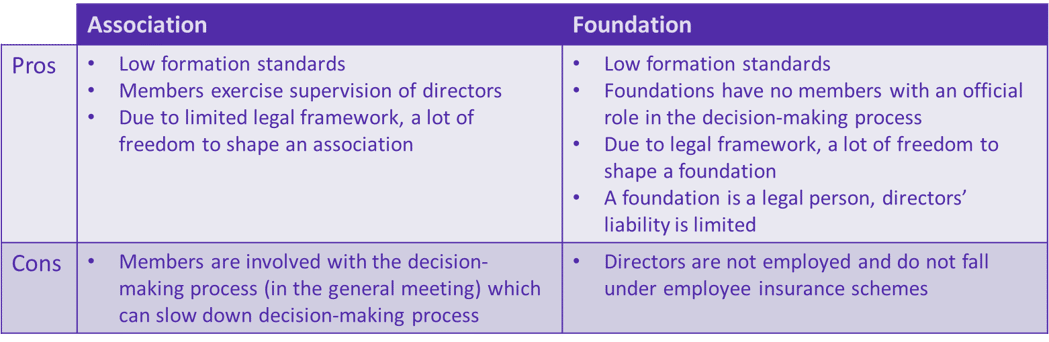
- Finally, the legal framework of different possible successor sustainable entities varies per country within the EU making it very complex for the legal partners within the IMI/IHI consortia to identify the best legal form. Therefore, a centralised EU-based legal advice service would help the project by sharing knowledge on the available successor forms and their pros and cons in different countries. At ttopstart we used the interviews and literature research to make a preliminary comparison between the two most common legal forms for such EU projects: an association and a foundation.
Table 2. Association vs foundation: differences and pros and cons
The insights listed above provide some background into the lessons learnt from IMI project coordinators. The next steps in our research will include identifying measurable parameters, e.g., average months for setting up a foundation, average administrative costs, as well as qualitative aspects such as how well the procedures are described, aspects to consider when selecting the legal base of a sustainable form, how the governance form in a specific country can be adapted case by case to facilitate the transition from the IMI/IHI project, the type of support from national platforms and best practices.
Help from ttopstart
How can ttopstart use these insights to support you in developing, managing and sustaining your IHI project? Get in touch in touch with us. By staying up-to-date on the landscape of IHI projects we continuously improve our own methodology to provide the best service for our clients: co-developing sustainable plans for their collaborative innovation projects.
References
- Goldman, M. et al. Getting Digital Assets from Public–Private Partnership Research Projects through “The Valley of Death,” and Making Them Sustainable. Mak. Them Sustain. Front. Med 5, 65 (2018).
- Creative Membership and Sponsorship Structures – Boardroom. https://boardroom.global/creative-membership-and-sponsorship-structures/ (2017).