The COVID-19 outbreak has immediate implications for the life sciences sector. We interviewed 50 European life science companies on how they are impacted by and have responded to the crisis. It is our goal to ensure that companies with impactful innovations can successfully navigate the pandemic and emerge not only as a survivor, but as a thriving business. We highlight the key challenges that life science companies specifically face today and we define a series of recommendations and suggestions to mitigate these problems. In this blog we provide a summary of the insights and a link to the full report.
Severe delays in R&D and clinical trials, but mixed impact on exit value
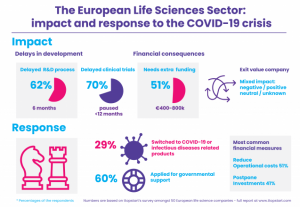
Nearly two-thirds of the companies interviewed expect delays in the development of their lead product due to the COVID-19 crisis. The average expected delay is 6-months. For those involved in clinical trials, 70% face delays, most of them paused or delayed up to 12-months. The slowdown of biomedical innovations may have a big impact the healthcare system, as well as on patients individually. These consequences will come on top of the already tensed healthcare system, in which shutdowns force waiting lists to grow rapidly.
About half (51%) of the companies expect to require extra funding. Most of the firms in need for additional funding require an amount between €100.000-€400.000, and it is not rare for companies to require an amount of even more than €800.000. Interestingly, these data also imply that an equal amount of companies does not face a financial setback.
Our data shows that the impact of the exit value of companies is very mixed; A quarter of the companies expect their exit value to be influenced negatively whereas about an equal amount of them expects a positive effect, no effect, or find it hard to make a prediction. Of the firms older than 11-years 21% are at risk of default (compared to 42% of younger companies) and only 36% of them require extra funding due to the COVID-19 crisis (compared to 63% of younger companies). This may indicate that older firms have a higher chance of surviving this crisis.
Strategic moves
A wide range of strategic moves in response the crisis are being made. Amongst financial strategies, reduction in operational costs and postponing investments are the most common. Interestingly, nearly a third of the interviewees have responded to the pandemic by switching to infectious diseases or other COVID-19 related areas.
Many companies are forced to revise their R&D and funding strategies while facing future uncertainties. The long-term effects of postponing investments could be the most detrimental for biomedical innovation. We plan on further elucidating which changes in market-entry strategies and validation strategies will be proven most successful.
Use of governmental support and sources for funding
Nearly 60% of the companies applied for the governmental support measures offered. Interestingly, only a quarter of the respondents in need of extra funding attempted to raise this via EU grants.
”We expect to raise additional funds by negotiating with current investors and new investors, comprising VC’s and industrial biotech investors. We will keep on raising fund through online channels”.
Extension of patents and simplify procedures
A 6-month extension of all European medical patents is a widely supported solution for the problem life science companies are facing today. We advise to look into the feasibility of such a measure. As many respondents acknowledged facing issues to add experiments and samples, we recommend extending and simplify filing and granting of procedures around clinical trials.
“We are facing a delay in market introduction and this would help (although not entirely solve the problem). It seems a sensible and realistic solution as long it is applied consistently.”
ttopstart
At ttopstart it is our mission to transform life healthcare and life sciences to accelerate impactful innovations. We have observed that life science companies specifically benefit from support in redefining their R&D strategy and in timely finding new investors and partners to anticipate to the corona-induced, novel situation. Our highly qualified team of consultants is specialized in co-creating competitive strategies throughout the entire life cycle. We have developed a platform to make it easier for biomedical innovators to keep track of updates on the newest support measures and funding opportunities related to COVID-19.
Read the full report here.
“The COVID-19 outbreak has immediate implications for the life sciences sector.”