Got questions? Find answers to commonly asked questions about IHI.
Why is the EU going from IMI to IHI?
IHI builds on what worked well in IMI, addresses the lessons learnt, and leverages the benefits of cross-sectoral collaboration in research and innovation to better respond to current and emerging health needs.
Its number one goal is to translate health research and innovation into tangible benefits for patients and societies.
In practice, all of this means that while some elements will stay the same, others change significantly.
As in IMI, the EU will provide 50 % of the funding for IHI, and the industry members will contribute the other 50%, primarily through ‘in-kind’ contributions. The total budget is 2.4 billion euros.
Which industry partners are involved in IHI?
As was the case in IMI, the ‘public’ member in the partnership is the European Union, represented by the European Commission.
The industry members are COCIR, EFPIA (including Vaccines Europe), EuropaBio and MedTech Europe, taking IHI beyond the pharmaceutical industry and bringing on board the medical technology, biotechnology, digital health and vaccine industries.
In addition, organizations that want to support specific research areas without becoming full members of IHI can apply to become ‘contributing partners’ (similar to the Associated Partners in IMI2).
Will IHI be governed differently from IMI?
New under IHI is the Science and Innovation Panel (S&IP), an advisory body that will bring together representatives of the scientific community and the wider health sector, such as regulatory bodies, patients and end-users. The panel will also include representatives of the European Commission and the industry partners of IHI and SRG members. The panel may also invite additional ad-hoc experts to join in discussions of specific subjects.
Like IMI, IHI has a Governing Board composed of equal numbers of representatives from the European Commission and the industry partners, plus a States Representatives Group (SRG) comprising representatives of the EU Member States plus countries associated with Horizon Europe.
What is the role of the new Science and Innovation Panel (S&IP)?
The S&IP will provide science-based advice to the Governing Board on scientific and technological matters such as:
- IHI’s draft work programmes, and in particular the draft call topics;
- updates to the Strategic Research and Innovation Agenda (SRIA);
- the planning of additional activities;
- the set-up of advisory groups focused on specific scientific priorities;
- synergies with other Horizon Europe activities, including other European partnerships and other EU and national programmes.
More about S&IP procedures, its members and meetings (IHI website)
When will IHI calls be published, and on which topics?
The end of October 2023, IHI published draft topics for IHI calls 6 and 7:
IHI call 6 (Two-stage topics)
- Support healthcare system resilience through a focus on persistency in the treatment of chronic diseases
- Development of practical guidance and recommendations for using real world data/real world evidence in healthcare decision-making
IHI Call 7 (Single-stage topics)
- Improving clinical management of heart disease from early detection to treatment
- User-centric technologies and optimised hospital workflows for a sustainable healthcare workforce
- Clinical validation of biomarkers for diagnosis, monitoring disease progression and treatment response
These opics are under consideration for inclusion in the next IHI calls for proposals, which IHI plans to launch in early 2024.
For more information per topic visit the IHI website
How do I write a successful proposal?
Writing a proposal and finding the right partners takes a lot of time and effort. In our brand-new Horizon Europe magazine, you will find a step-by-step roadmap, which will guide you in writing a successful IHI proposal in four phases. Our advice: start now! See the previous question for information on the draft topics in IHI.
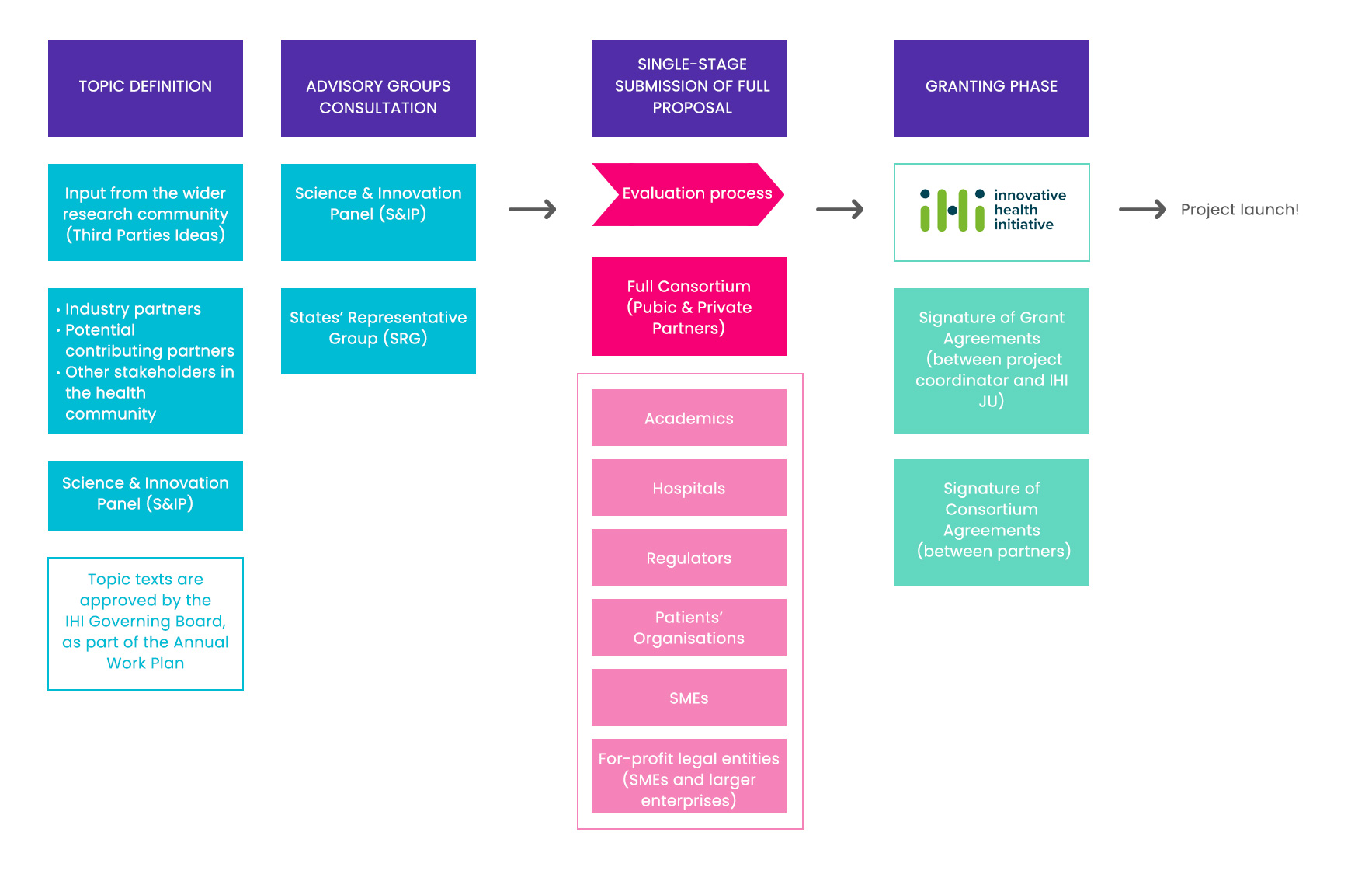
When will a single-stage call be eligible for evaluation?
The one-stage procedure is a bottom-up approach that allows much more flexibility than the 2-stage proposals. The call text is written by IHI/Science and Innovation panel (S&IP) and not by industry partners. So, public partners and Industry write the application together from the beginning.
Partner organizations could be academics, hospitals, public bodies, regulators NGOs, research organizations, for-profit legal entities (SME and larger enterprises), Patients’ Organizations and intergovernmental organizations. established in a member state or Associated Country. New under IHI: organizations can also be based in Low and Middle-Income Countries (see Horizon Europe list)
Important: at least 45% in-kind contribution of the total project costs needs to come from the consortium. Proposals not meeting this criterion will not be considered for evaluation!
How many single-stage proposals will IHI fund?
Based on our current information, several proposals will be funded (in contrast with two-stage calls). However, the final number will depend on the available budget for single-stage calls.
‘Bringing researchers, entrepreneurs and stakeholders together to work on sustainable solutions in healthcare. That is what we do. And that will be the big challenge in IHI. How to find the right partners? How do you align your goals with those of others? My appeal: start on time! We are already discussing with some of our strategic accounts how we are going to build the ideal consortium.’
– TTOPSTART DIRECTOR SIETSKE ZAGERS
Related articles
IHI launches new funding round: calls 6 and 7
IHI launches new funding round: calls 6 and 7The Innovative Health Initiative (IHI) has launched two new calls for proposals, IHI calls 6 and 7, addressing a wide range of challenges in health research and innovation.Approximately EUR 120 million in funding from IHI...
Registration open for IHI Call Days calls 6 and 7
Registration is open for IHI Call Days calls 6 and 7Save the date!Registration is now open for the IHI Call Days featuring calls 6 and 7, accessible through the IHI Call Days platform. Information sessions Information sessions are scheduled from January 10 to 16,...
IHI launched new draft topics for calls 6 and 7
IHI has published draft topics for IHI calls 6 and 7The draft texts of the topics planned for inclusion in IHI's next call for proposals are now available online. The following topics are currently under review for potential inclusion in the upcoming IHI calls for...
IHI launched two new calls for proposals
IHI has launched two new calls for proposalsOn 27 July 2023, the Innovative Health Initiative (IHI) has officially launched the calls 4 & 5. The new calls for proposals features topics on the environmental impacts of healthcare, addressing the use of animals in...